Ions form when atoms lose or gain electrons to obtain a full outer shell. Metals are situated on the left side of the periodic table.
Chemical Bonding Ionic Bonds Ionic Bonds Are Made Between Metal And Non Metal Atoms Electrons Are Transferred From The Metal Atom To The Non Metal Atom Ppt Download
An ion is an atom or group of atoms with a positive or negative charge.
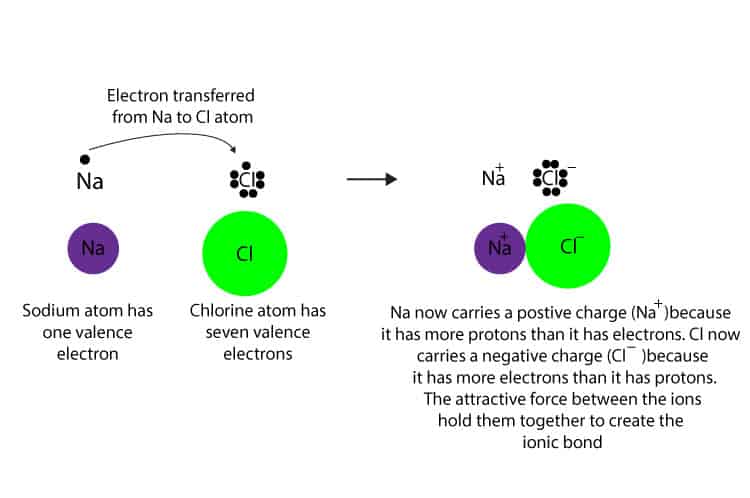
. Formation of ionic compounds When metals react with non-metals electrons are transferred from the metal atoms to the non-metal atoms forming ions. The type of ions that metals form are called positively charged ions. Learn vocabulary terms and more with flashcards games and other study tools.
Atoms of two or more elements can combine to form a compound in which the atoms are not changed to different types of atoms or fractions of atoms. Do metal forms form ions or anions. 30 Votes Halogens always form anions alkali metals and alkaline earth metals always form cations.
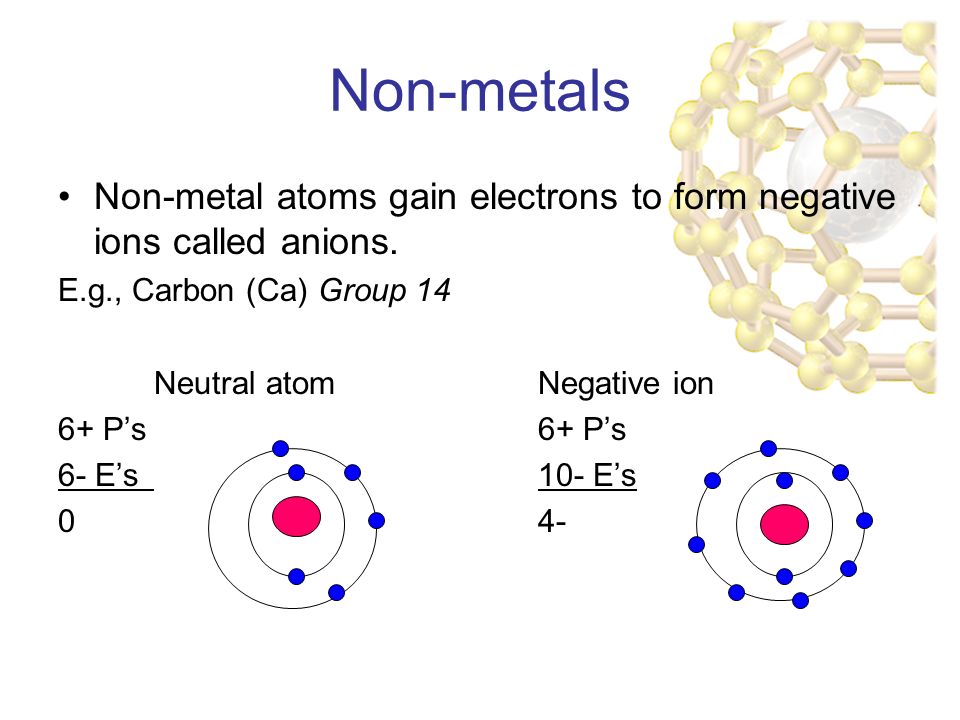
Halogens always form anions. They form ions by donating extra electrons to another electron-deficient element. Atoms and Atomic Structure.
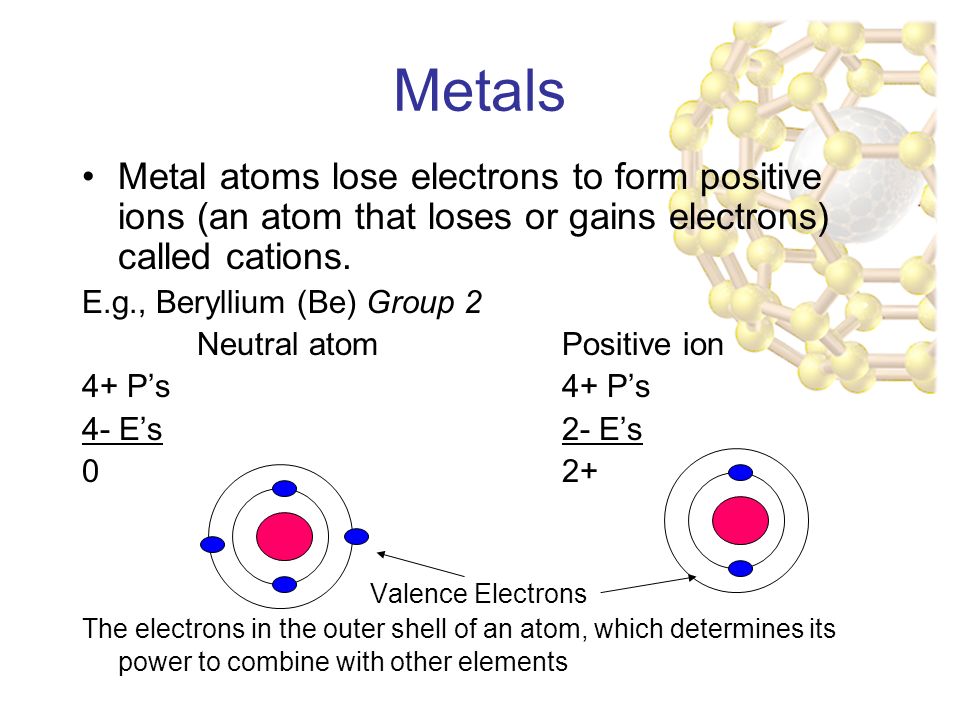
Positively charged sodium and aluminium ions. Atoms and Atomic Structure. Metal atoms become positively charged ions because they lose electrons when they react with another atom.
Want this question answered. Metals that can form cations with variable charge ar typically found in which of the following areas of the periodic table. A group 3A metal charge is.
Metals usually form positive ions because metals are very electropositive. These elements all have valence electrons in an s orbital. The relative number of atoms of each element in a given compound is always the same and is a characteristic of the compound.
Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Metal atoms lose the electron or electrons in their highest energy level and become positively charged ions. Positively charged ions are called _____ while negatively charged ions are called _____.
Metal atoms lose electrons to form positively. Metals form electopositive ions or cations. What type of ions do metals form and why.
Iron silver nickel whilst most other nonmetals typically form anions eg. A group 2A metal charge is. 475 1695 Views.
What is a group of the same type of atoms called. The scientific name for positively charged ions is cations. Metals form positive ions and nonmetals negative ions Explanation.
Be notified when an answer is posted. Groups 1 and 2 are called the alkali metals and alkaline Earth metals respectively. - Group 7A - Group 4A - Group 2A - Transition metals - Group 4A - Transition metals.
A nonmetal usually form an anion. So when electronegative atoms atoms which tend to suck-in electrons react with metal atoms they tend to form ionic compounds consisting of anions negative ions of the electronegative element. See answer 1 Best Answer.
Atoms are neither created nor destroyed in a chemical reaction. Non metals usually form what type of ions. Group 1 and group 2 are called the alkali metals and alkaline earth metals.
Click to see full answer Keeping this in view what type of elements form anions. That means that the outer electrons of each atom of a metal are very loosely bound to the atoms nucleus. Most other metals form cations eg.
Groups 1 and 2 are called the alkali metals and alkaline Earth metals. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Start studying Elements compounds ions atoms and molecules.

Learn The Difference Between Ionic And Covalent Bonds See Examples Of The Two Types Of Ch Ionic And Covalent Bonds Covalent Bonding Covalent Bonding Worksheet

Why Do Metals Always Form Positive Ions Quora

How Do Atoms Form An Ionic Bond
Chemical Bonding Ionic Bonds Ionic Bonds Are Made Between Metal And Non Metal Atoms Electrons Are Transferred From The Metal Atom To The Non Metal Atom Ppt Download
0 Comments